Our Last 5 Publications:
Tripartite Hydrogen-Bonding as a Driving Force for High-Concentration Cyclization of Poly(L-lactide)
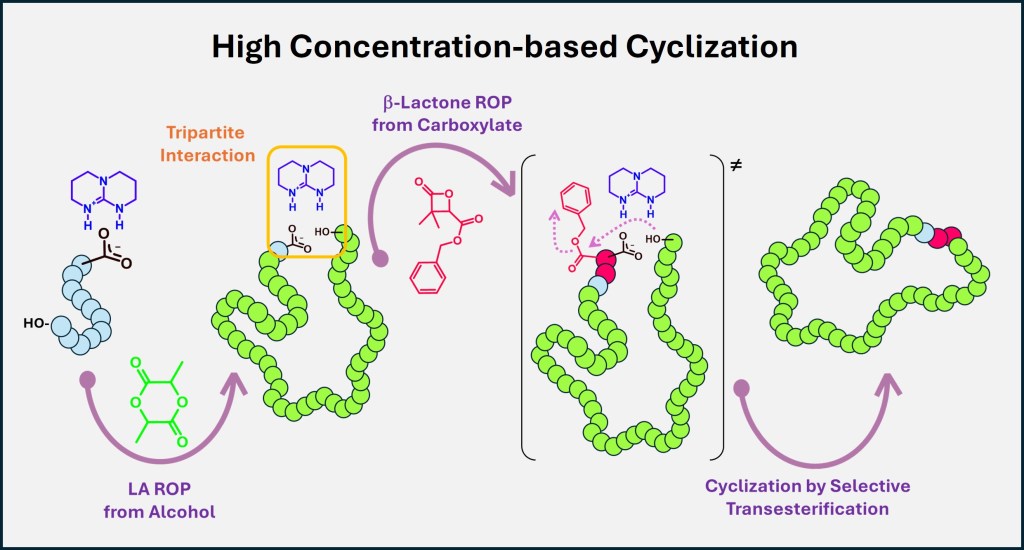
Here, we report a three-step strategy enabling efficient cyclization of high-Mn PLLA at 0.5 M. Key to success is a persistent tripartite complex driven by hydrogen bonding and ion pairing. Selective intramolecular transesterification at a benzylic ester triggers ring closure, affording cyclic PLLA (Mn ≈ 26,000 g/mol) under synthetically practical conditions. SPM imaging reveals nanorings with diameters consistent with the expected contour length of cyclic PLLA chains.
Selective Low-Temperature Depolymerization of Highly Transesterified P(LLA-co-CL) Copolymers: Efficient Lactide Recovery and PCL Upcycling
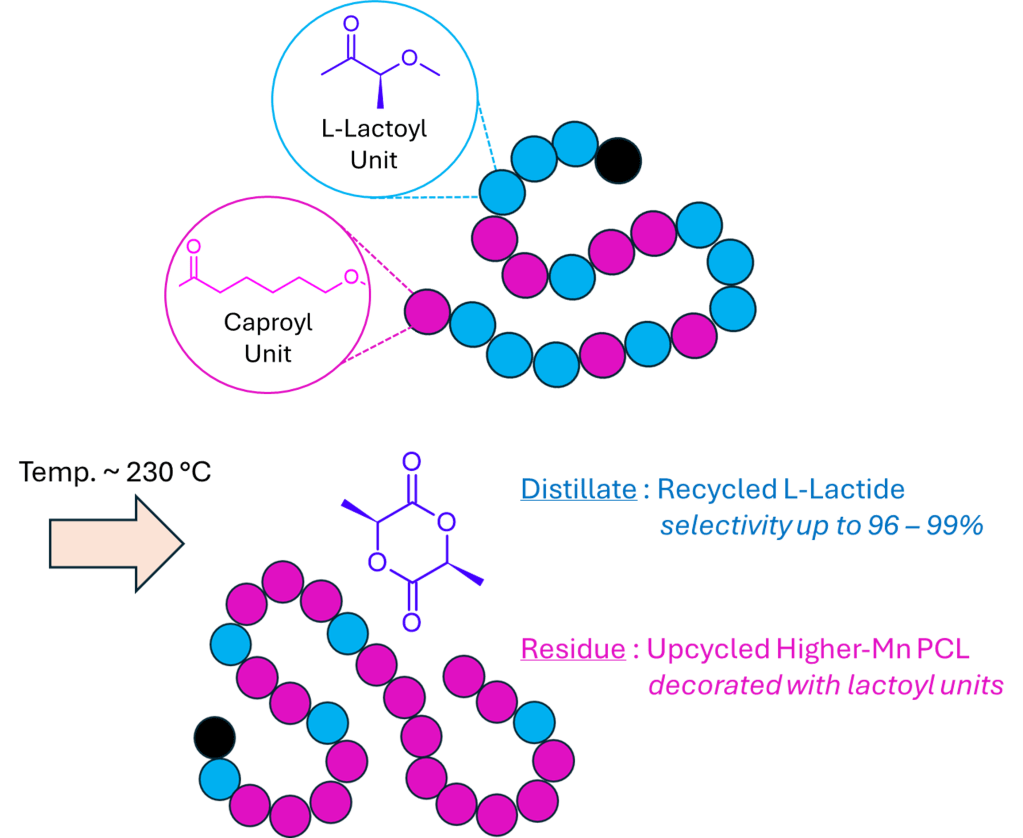
Here we show that highly transesterified poly(L-lactide-co-caprolactone) (P(LLA-co-CL)) copolymers undergo low-temperature depolymerization with exceptional selectivity for L-lactide (LLA). Upon vacuum distillation at 230 °C, distillates are recovered that are highly enriched in LLA (up to 96-99 mol%), while the polymer residues reorganize into higher-molar-mass polycaprolactone (PCL) chains sporadically decorated with lactoyl units. The recycling process combines the selective regeneration of virgin-quality LLA with the generation of unprecedented “upcycled PCL” architectures, distinct from conventional PCL and offering new opportunities for property design.
Reevaluating Lithium Chloride as a Safer Catalyst for Polylactide Synthesis: A Toxicological and Process Perspective
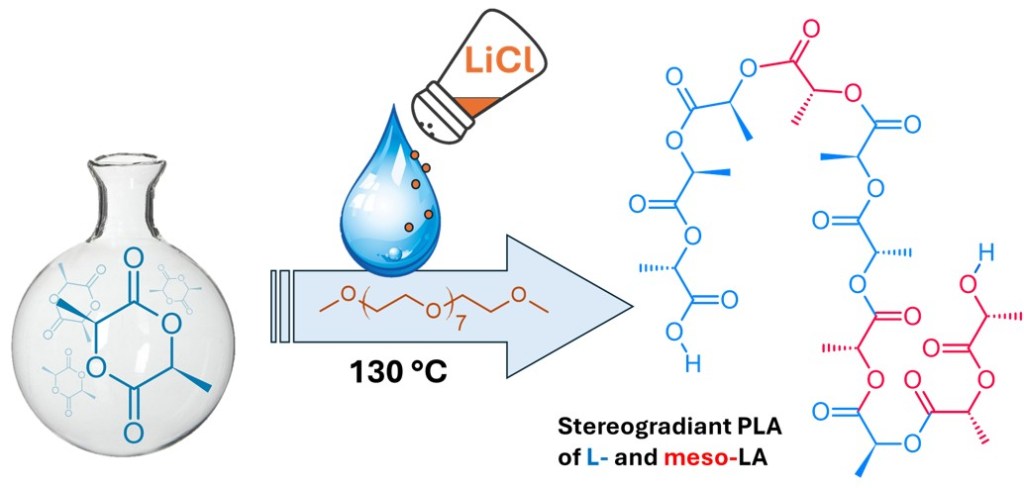
We report a reinvestigation of LiCl as a catalyst for the ROP of L-lactide, using octa(ethylene glycol) dimethyl ether (EG8) as a coordinating agent. The presence of trace water does not impair polymerization efficiency, eliminating the need for high-vacuum techniques or aggressive drying protocols. Water initiates the reaction via in situ formation of HO-Li+ species, which generate lithium lactate, the actual active species in the system. The Li+ coordination environment, modulated by EG8, governs both reactivity and stereocontrol, with prolonged reaction leading to partial epimerization of L-lactide into meso-lactide.
From Polyethylene to Conjugated Polyenes: A Mechanochemical One-Pot Upcycling Strategy for Selective Functionalization
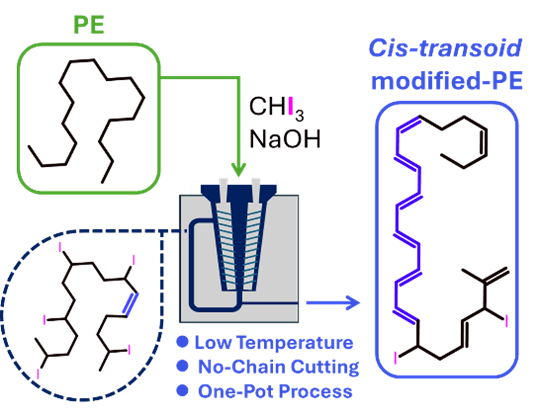
Here, we report a solvent-free, mechanochemical approach to introduce conjugated polyene sequences into polyethylene (PE) via a twin-screw extrusion process. The controlled iodination/elimination strategy enables the stereoselective formation of cis-transoid polyenes, a configuration typically achieved under cryogenic conditions but here accessible at high temperatures (106 °C) in a melt-processing environment. This work presents a scalable route for the selective functionalization of polyolefins, opening new avenues for their valorization into advanced materials and demonstrating the feasibility of high-temperature processing for the formation of conjugated polyenes.
CO2-Binding Alcohols as Potential Candidates for Poly(Vinyl Chloride) Upcycling
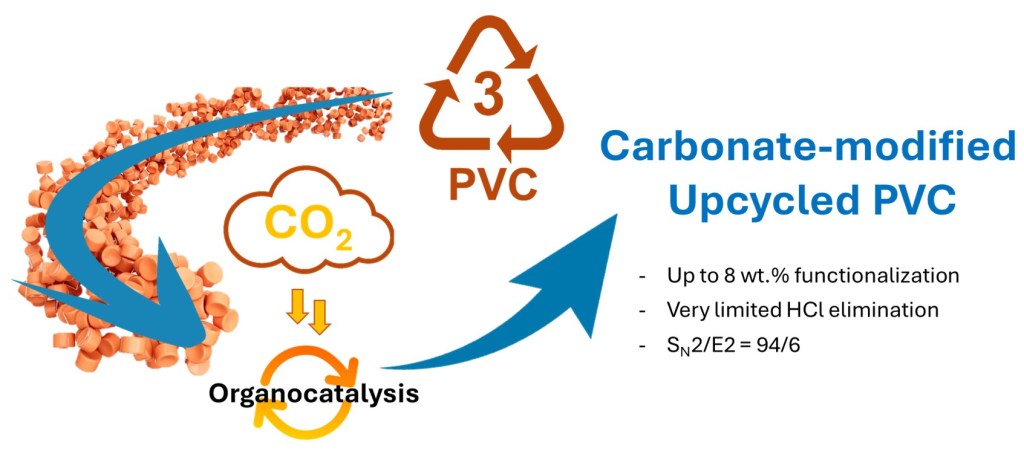
PVC is tough to recycle – its high chlorine content and resistance to standard methods make it a real challenge. Our work tackles this head-on using CO2-binding alcohols (CO2BALs) derived from TBD to upgrade PVC through smart functionalization. We achieved a sharp SN2/E2 selectivity of 94/6 and confirmed successful carbonate grafting. This project paves the way for greener, more efficient PVC upcycling, and a fresh use for captured CO2.
